Checklist for Verifying Supplement Claims
Supplements are not FDA-approved for safety or effectiveness before hitting the market. This means the responsibility to evaluate their claims falls on you. Misleading labels, harmful interactions, and unverified ingredients are risks you need to navigate. Here's how you can protect yourself:
- Read Labels Carefully: Check for required elements like the Supplement Facts panel, ingredient list, and manufacturer details. Watch out for proprietary blends and missing disclaimers.
- Spot Illegal Claims: Claims to "cure" or "treat" diseases are red flags. Look for the FDA disclaimer: "This statement has not been evaluated by the Food and Drug Administration..."
- Verify Third-Party Testing: Look for certification seals like USP Verified or NSF. Request a Certificate of Analysis (COA) for batch-specific quality checks.
- Inspect Packaging: Ensure tamper-evident seals are intact and check expiration dates. Damaged packaging can signal contamination.
- Evaluate Scientific Evidence: Use trusted sources like PubMed to confirm claims are backed by human studies, not vague or exaggerated marketing.
Shortcut the process with tools like SlipsHQ, which scans barcodes for ingredient purity, certifications, and safety alerts in seconds.
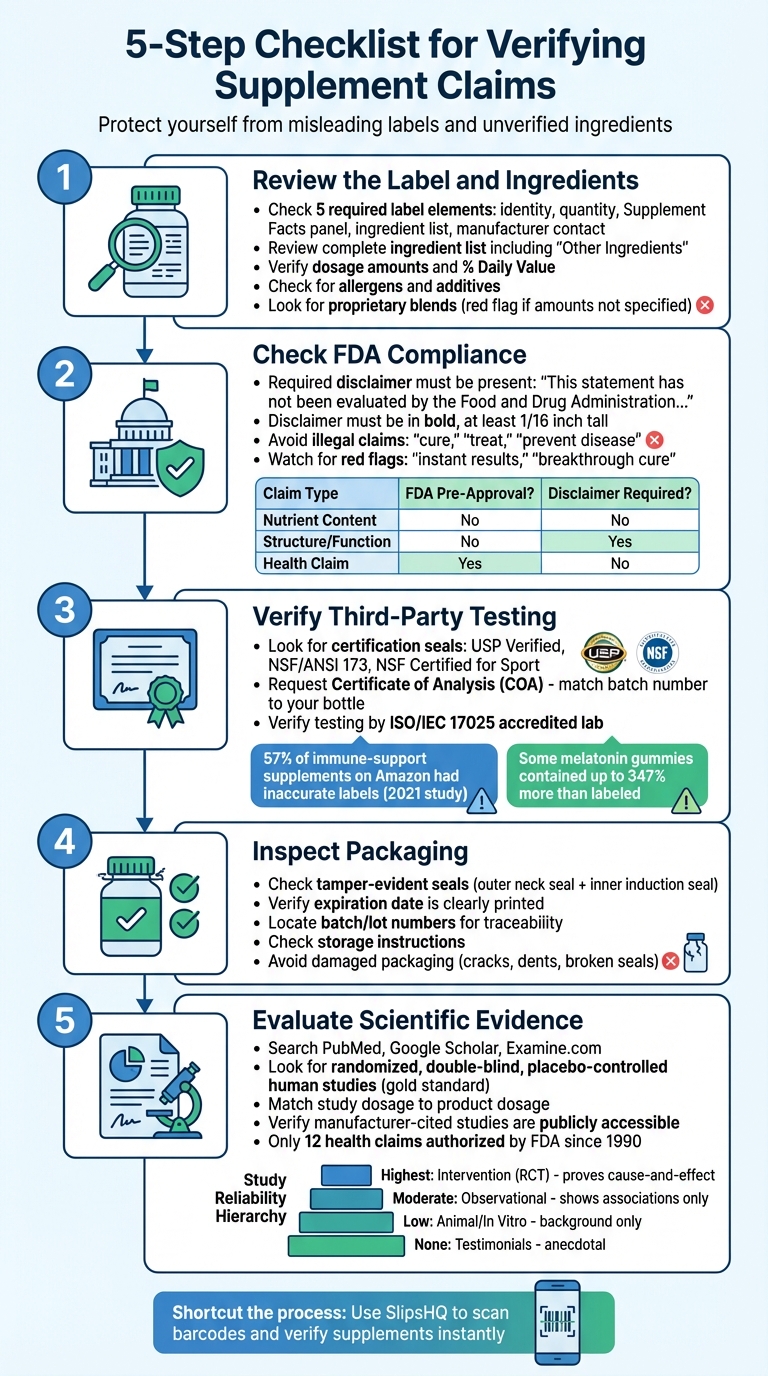
5-Step Checklist for Verifying Supplement Safety and Claims
Step 1: Review the Label and Ingredients
In the U.S., supplements are required to include five key label elements: a statement of identity (like "Vitamin C Supplement"), the net quantity of contents, a Supplement Facts panel, a full ingredient list, and the manufacturer's contact information. If any of these are missing, it's a warning sign. These details are essential for assessing whether a supplement is trustworthy.
Read the Complete Ingredient List
The Supplement Facts panel is your go-to for important details such as serving size, the number of servings per container, and the amount of each dietary ingredient per serving. Be sure to also check the "Other Ingredients" section, which lists fillers, binders, preservatives, and flavorings in descending order by weight.
Pay close attention to proprietary blends. These often only show the total weight of the blend without specifying the amounts of individual ingredients, which can make it harder to understand exactly what you're getting.
Confirm Dosage Amounts
The Supplement Facts panel includes the "% Daily Value" (% DV) for nutrients, which tells you how much of the recommended daily intake is provided by one serving. For a product to claim it is "high" in a nutrient, it must provide at least 20% of the Daily Value per serving. A "good source" claim requires 10% to 19%. For ingredients without an established Daily Value - like certain herbs or amino acids - the label should include the note "Daily Value Not Established." Always cross-check claims on the front of the package with the actual numbers in the Supplement Facts panel.
Check for Allergens and Additives
By law, U.S. manufacturers must clearly list major allergens on supplement labels. Look closely at the "Other Ingredients" section for allergens such as soy, dairy, shellfish, or tree nuts, especially if you have sensitivities. Also, take note of artificial colors, preservatives, or stabilizers that you might want to avoid. For herbal supplements, make sure the label specifies the plant part used, such as root, leaf, or seed.
If the label includes a structure/function claim like "supports heart health", it must also display the FDA-required disclaimer: "This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease". This disclaimer signals that the claim has not been verified by the FDA. Once you've thoroughly reviewed the label, you're ready to move on to Step 2 to check for regulatory compliance.
Step 2: Check FDA Compliance and Regulatory Requirements
The FDA has distinct regulations for supplements and drugs, so it's essential to follow these guidelines to separate acceptable claims from prohibited promises. Additionally, specific labeling elements must meet FDA standards.
Required Disclaimer Statements
If a supplement makes a structure/function claim - such as "supports immune health" or "promotes healthy digestion" - it must include the exact FDA-required disclaimer:
"This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease."
This disclaimer is mandatory for structure/function, general well-being, and nutrient deficiency claims. It must be printed in bold type, with letters at least 1/16 inch tall. Typically, the disclaimer is placed either immediately next to the claim (with no intervening text) or elsewhere on the same label panel, linked to the claim by an asterisk and enclosed in a box. Missing or improperly formatted disclaimers are a clear compliance issue.
Identifying Misleading or Illegal Claims
Dietary supplements are not allowed to claim they can "diagnose, treat, cure, or prevent any disease." Phrases like "cures COVID-19", "treats diabetes", or "prevents heart disease" classify the product as an unapproved drug, which is illegal under FDA rules. Similarly, terms like "instant results" or "breakthrough cure" often indicate fraudulent products and should raise red flags.
Be wary of implied claims as well. For instance, a product might list ingredients associated with nitric oxide production and subtly suggest it benefits blood vessels - without sufficient evidence to back it up. Other warning signs include poor-quality printing, spelling mistakes, or missing contact information, all of which could signal fraud.
Understanding Claim Categories
To ensure compliance, it's important to know the three main types of claims the FDA recognizes for supplements, as each has specific rules:
| Claim Type | Definition | FDA Pre-Approval Required? | Mandatory Disclaimer Required? |
|---|---|---|---|
| Nutrient Content Claim | States the level of a nutrient (e.g., "High in Vitamin C", "Low fat") | No (must meet defined levels) | No |
| Structure/Function Claim | Describes how a nutrient supports normal body structure or function | No | Yes |
| Health Claim | Explains the link between a substance and reduced disease risk | Yes | No (though a qualified disclaimer may be needed) |
Reminder: Verify that each claim aligns with FDA definitions and requirements to avoid compliance issues.
Step 3: Verify Third-Party Testing and Certifications
Third-party testing serves as an independent check to ensure that a supplement lives up to its label claims. Unlike testing done by the manufacturer, third-party labs have no financial interest in the product’s success. They verify the supplement’s potency, purity, and ingredient accuracy, offering an unbiased assessment of quality.
Look for Third-Party Certification Seals
Certification seals on product packaging can help you quickly identify supplements that meet rigorous quality standards. Some of the most recognized programs include USP Verified, NSF/ANSI 173, and NSF Certified for Sport. Each focuses on different aspects of quality:
| Certification Seal | Primary Verification Focus | Best For |
|---|---|---|
| USP Verified | Potency, purity, ingredient breakdown (dissolution), and GMP compliance. | General consumers seeking quality assurance. |
| NSF/ANSI 173 | Label accuracy, contaminant review, and toxicology. | Standard quality verification. |
| NSF Certified for Sport | Screens for banned substances and undeclared stimulants/steroids. | Professional and amateur athletes. |
For instance, USP Verified ensures that a supplement dissolves properly so its ingredients can be absorbed as intended. Meanwhile, NSF conducts independent testing, including annual audits and periodic retesting, to confirm ongoing compliance. If you see these seals, it’s a good idea to request additional documentation to further verify the product’s quality.
Ask for Certificates of Analysis
A Certificate of Analysis (COA) is a document provided by a quality assurance team or third-party lab that confirms whether a specific batch meets its stated quality, purity, and potency standards. To get a COA, you can usually contact the manufacturer directly - most offer this information via their website or customer service.
When reviewing a COA, make sure the batch number matches the one on your supplement bottle. Check that the test results meet acceptable standards, and confirm that the testing was performed by an ISO/IEC 17025 accredited lab. Also, ensure the document is signed and dated by an authorized quality assurance representative. Keep in mind, though, that a COA reflects the quality of a single batch and doesn’t replace a comprehensive quality system.
Match Label Claims to Test Results
To dig deeper into a supplement’s claims, many certification programs offer searchable online databases where you can confirm whether a product is currently certified. Some organizations, like Labdoor and the International Fish Oil Standards (IFOS), even publish detailed lab results online, letting you compare label claims with actual test data.
For example, programs like Informed Sport test every batch of a product before it’s released, and the NSF Certified for Sport app allows you to scan or search for specific lot numbers to confirm that your exact bottle was tested. This level of transparency is crucial. A 2021 study of 30 immune-support supplements sold on Amazon found that 57% had inaccurate labels, with 13 products missing key ingredients like aloe vera or elderberry. Similarly, research on 25 melatonin gummy brands revealed that some contained up to 347% more melatonin than what was listed on the label. These examples highlight why verifying test results is so important.
Step 4: Inspect Packaging and Product Condition
Carefully inspecting supplement packaging is crucial for ensuring both quality and safety. Damaged or suspicious packaging can indicate issues like tampering, improper storage, or even counterfeit products. Once you've reviewed the product's claims and any third-party testing results, take a close look at the packaging itself to confirm its integrity. This step works hand-in-hand with label checks and testing to give you a complete picture of the product's quality.
Check Seals and Expiration Dates
Start by examining the tamper-evident seals. Legitimate supplements typically have two layers of protection: an outer neck seal (often clear or branded plastic) and an inner induction seal under the cap. Both seals should be intact and show no signs of tampering, such as being broken, loose, or resealed. If either seal appears unusual or inconsistent with the brand's typical packaging, that's a warning sign.
Next, check the expiration date. While the FDA doesn't mandate expiration dates on supplement labels, most reputable manufacturers include them to guarantee product potency. Make sure the date is clearly printed and hasn't been altered. Additionally, inspect the label quality - poor printing, smudges, or errors can be immediate indicators of a counterfeit or compromised product.
Locate Batch Numbers and Storage Instructions
Packaging also provides critical information for tracking and usage. Look for batch or lot numbers, which are essential for traceability. These numbers allow manufacturers to track specific production runs and let you verify a Certificate of Analysis (COA) tied to that batch. Ensure the batch numbers are clearly visible and easy to read.
Another important detail to check is the storage instructions. Labels should include directions for use, any necessary cautions, and storage guidelines. Supplements exposed to heat, moisture, or light can deteriorate, reducing their effectiveness. The FDA requires that supplement labels display five key elements: the product's identity, net quantity, nutrition labeling (Supplement Facts), ingredient list, and the manufacturer’s name and location.
Look for Damaged Packaging
Damaged packaging is a major red flag. Cracks, dents, or broken seals can expose the product to air or moisture, which can degrade the active ingredients or lead to contamination by bacteria, mold, or yeast. Even minor damage can compromise the product's safety and effectiveness. If you notice any signs of damage, it's best to avoid that product and opt for a safer alternative.
Step 5: Evaluate Scientific Evidence
Once you've reviewed labels and testing results, the next step is to dig into the scientific evidence behind the claims. This is where you separate reliable, science-backed products from those that rely on flashy marketing. Understanding how to assess scientific research ensures you're making well-informed decisions about what you consume. Think of it as connecting product testing with real-world clinical relevance.
Find Research Supporting the Claims
To start, explore specialized research databases. Websites like Examine.com provide detailed evidence reviews, while Google Scholar and PubMed offer access to peer-reviewed studies. For instance, Examine.com's "Supplement Navigator" breaks down efficacy, safety, and dosage information tailored to specific health conditions.
The gold standard for evidence is randomized, double-blind, placebo-controlled human intervention studies. These studies are the most reliable for establishing cause-and-effect relationships. While animal and test-tube studies can provide useful background, they don't directly support claims about human health. Also, make sure the study participants match your demographic. For example, research on memory loss in elderly adults isn't evidence for benefits in healthy children.
Distinguish Vague Claims from Specific Claims
Not every supplement claim demands the same level of proof. The FDA classifies claims into different types, and knowing these distinctions can help you spot potential issues. For example, health claims like "reduces risk of osteoporosis" require significant scientific agreement and FDA authorization. Since 1990, only 12 health claims have been authorized, reflecting the rigorous evidence needed.
On the other hand, structure/function claims - such as "supports bone health" - don’t need pre-approval but must still be truthful and supported by evidence. These claims must include the FDA disclaimer: "This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease". If you don’t see this disclaimer, it’s a major red flag.
General claims like "boosts energy" or "supports immunity" are harder to verify compared to measurable outcomes. The FTC, for instance, flags certain weight loss claims - like promises of permanent weight loss without diet or exercise - as misleading and a sign of false advertising.
Verify Manufacturer-Cited Studies
When manufacturers cite research, take a close look. Check that the dosage in the study matches the amount listed on the product label. A study using 500 mg of an ingredient cannot justify claims for a product containing just 50 mg.
Also, confirm that the study outcomes align with the claims being made. For instance, a study showing a temporary boost in metabolism doesn’t prove the product "promotes weight loss". As the FDA emphasizes, strong health advice is based on consistent research over time, not a single study. The most reliable evidence comes from multiple trials or systematic reviews.
Be cautious of these red flags in manufacturer-provided research:
- Illegal disease claims: Supplements cannot claim to diagnose, treat, cure, or prevent diseases.
- Proprietary or unpublished research: Credible claims should be backed by publicly accessible studies.
- Nutrient content mismatches: For example, a claim of being "high in" a nutrient requires at least 20% of the Daily Value per serving, while "good source" needs 10-19%.
An example of evolving evidence comes from October 2017, when the FDA proposed revoking the health claim about soy protein reducing coronary heart disease risk. After reviewing all available scientific evidence, the agency determined the claim no longer met the "Significant Scientific Agreement" standard. This highlights why staying updated on the latest research is crucial.
| Study Type | Reliability for Claims | What It Can Prove |
|---|---|---|
| Intervention (RCT) | Highest | Can establish cause-and-effect |
| Observational (Cohort) | Moderate | Shows associations, not causation |
| Animal/In Vitro | Low | Provides background but no human proof |
| Testimonials | None | Anecdotal; often placebo or coincidence |
Understanding these study types is essential for verifying claims and ensuring they align with earlier label and testing reviews.
Conclusion: Use SlipsHQ to Verify Supplements Faster
Manually verifying supplement claims can feel like a never-ending task. You might end up jumping between the FDA's database, searching certifier sites like USP or NSF by lot number, digging through research on PubMed, and comparing prices across retailers. It’s a time drain, and it’s easy to miss critical details - like hidden additives or sketchy pricing. That’s where SlipsHQ steps in to save the day.
SlipsHQ simplifies supplement verification with just a barcode scan. Instead of spending hours piecing together information, you instantly gain access to trust scores based on science, detailed ingredient purity data, third-party certification status, and real-time safety alerts. With a database covering over 200,000 supplements, the app pulls data from public records, research studies, and trusted certifiers like USP, NSF, and Informed Choice to ensure accuracy.
The app automatically flags issues like unverified additives, potential drug interactions, or missing certifications. Let’s say you’re shopping in-store: a quick barcode scan can reveal if a product lacks third-party testing or contains undeclared ingredients. Plus, its price comparison tool helps you spot counterfeit products by flagging unusually low prices compared to the manufacturer’s official listings.
Whether you’re checking dosages, scanning for allergens, or verifying scientific claims, SlipsHQ consolidates everything into one quick step. Its personalized supplement stack feature even helps you design a routine tailored to your health goals, built on evidence-based insights. With SlipsHQ, what used to take hours can now be done in seconds, giving you confidence in the supplements you choose.
FAQs
How can I spot misleading supplement claims?
To identify misleading claims on supplements, start by thoroughly examining the product label. A transparent ingredient list and third-party certifications like USP Verified or NSF Certified for Sport can signal reliable testing and quality standards.
Check the packaging carefully - issues like spelling errors, damaged seals, or overly generic claims might be warning signs. You can also use tools like the SlipsHQ app, which allows you to scan barcodes and access science-based reviews on a supplement’s safety, quality, and effectiveness. Staying informed helps you make smarter, health-focused decisions.
What should I check for in third-party supplement testing certifications?
When evaluating third-party testing certifications for supplements, prioritize those that confirm ingredient accuracy, verify the absence of harmful substances like heavy metals, pesticides, or bacteria, and ensure compliance with strict safety and quality standards. Trusted seals such as USP Verified, NSF Certified for Sport, or Informed Choice are key indicators of thorough testing and adherence to industry guidelines.
These certifications provide reassurance that the product's contents align with its label, offering greater confidence in both its safety and effectiveness.
Why is it important to check the scientific evidence behind supplement claims?
Checking the facts behind supplement claims is essential to make sure the products you choose are safe, effective, and properly labeled. Unlike prescription drugs, supplements don’t go through FDA pre-approval, leaving it up to manufacturers to ensure their products meet safety and quality requirements.
Looking into credible research and third-party testing can help you verify the purity, strength, and effectiveness of the ingredients. This not only safeguards your health but also empowers you to make smarter choices about what you’re consuming.
