GMP vs. Non-GMP: Impact on Supplement Safety

When buying supplements, the difference between GMP-certified and non-GMP products is crucial for your health and safety. GMP (Good Manufacturing Practices) certification ensures supplements are made under strict quality and safety standards, covering everything from ingredient testing to packaging. Non-GMP products, on the other hand, lack these safeguards, increasing risks like contamination, mislabeling, and inconsistent quality.
Key Takeaways:
- GMP Certification: Ensures consistent quality, accurate labeling, and reduced contamination risks.
- Non-GMP Risks: Higher chance of harmful contaminants, incorrect dosages, and unreliable results.
- Third-Party Certification: Some brands exceed basic GMP standards by obtaining additional certifications (e.g., NSF), offering extra assurance.
- Consumer Tools: Platforms like SlipsHQ let you scan barcodes to check safety, quality, and transparency with trust scores.
If you want safer supplements, look for GMP certification and use tools like SlipsHQ to verify product quality. These steps help you avoid unsafe options and make informed choices for your health.
What Is GMP Certification?
GMP certification for dietary supplements signifies that manufacturers follow strict standards for quality, safety, and processes in how products are produced, packaged, and stored. Every batch must meet specific criteria for identity, purity, strength, and composition. In the United States, the Food and Drug Administration (FDA) enforces legally binding current Good Manufacturing Practice (cGMP) regulations under 21 CFR Part 111. Beyond these mandatory rules, many manufacturers seek voluntary third-party certifications - such as those from NSF - to demonstrate they exceed the basic requirements. This system lays the foundation for understanding GMP's role in ensuring product safety and consistency.
Core Principles of GMP
At the core of GMP compliance is a structured quality management system that assigns responsibilities, establishes written procedures (standard operating procedures), and ensures detailed batch records are maintained. This documentation creates a traceable trail, making it easier to pinpoint and address issues if they arise.
Control over ingredients and testing plays a major role. Manufacturers must define clear specifications for the identity, purity, strength, and composition of all raw materials. They also set limits for potential contaminants like heavy metals, microbes, or pesticides. This process involves qualifying suppliers, verifying certificates of analysis, and conducting identity tests to ensure only high-quality ingredients are used.
GMP also enforces strict oversight of the manufacturing process. Steps such as weighing, blending, granulation, encapsulation, and packaging are closely monitored and documented to meet defined specifications. Regular equipment maintenance, calibration, and control of environmental factors - like temperature and humidity - further minimize risks. Measures like line clearance checks and physical separation are implemented to prevent cross-contamination.
Facility design is another critical aspect. Production areas must allow for effective cleaning, pest control, and prevention of cross-contamination. This includes using cleanable surfaces, optimizing layouts, and ensuring proper storage conditions. To maintain cleanliness, manufacturers follow rigorous sanitation programs and adhere to detailed cleaning schedules.
Lastly, personnel training is essential for successful GMP implementation. Staff involved in production must receive ongoing training in procedures, hygiene, and safety protocols. Well-trained employees play a key role in identifying deviations and ensuring that processes are consistently followed.
Why GMP Matters for Supplements
GMP certification helps ensure the safety and quality of dietary supplements by requiring rigorous testing of each batch. This includes identity verification, potency tests, microbial checks, and evaluations of physical characteristics like capsule integrity or tablet disintegration. Some manufacturers also perform stability studies to confirm that products remain safe and effective throughout their shelf life.
For consumers, one of the biggest advantages of GMP is better label accuracy. GMP practices ensure that the ingredients and their amounts match what is listed on the label, minimizing risks from incorrect dosages, mislabeling, or hidden contaminants. On the flip side, manufacturers without GMP compliance may face issues like inconsistent quality, incomplete testing, and poor production controls - problems that could result in ineffective products, unexpected side effects, or exposure to harmful substances.
While GMP certification isn't a guarantee of perfection, it acts as a critical safeguard, significantly reducing the chances of manufacturing errors. Choosing supplements from a GMP-certified manufacturer provides greater confidence in the quality and reliability of the product.
Key Differences Between GMP and Non-GMP Supplements
The difference between GMP-certified and non-GMP supplements plays a crucial role in determining product safety and effectiveness. Recognizing these distinctions explains why some supplements deliver reliable results while others may fall short - or even pose risks.
Safety and Contamination Risks
GMP-certified manufacturers follow stringent standards to verify the identity, purity, strength, and composition of every ingredient they use. This thorough process significantly reduces the risk of contamination from microbes, heavy metals, or undeclared substances. It also ensures that the product label accurately reflects what's inside. These rigorous testing protocols are the backbone of consistent product quality.
On the other hand, non-GMP products often skip or scale back critical testing steps, such as verifying raw materials, conducting process checks, or performing final product testing. This lack of oversight increases the likelihood of incorrect ingredients, improper dosages, or harmful contaminants. Without measures like hazard assessments or environmental monitoring, contamination issues may go unnoticed until they cause problems. For instance, a GMP-certified facility tests every batch of raw herbs for contaminants, while a non-GMP operation might rely solely on supplier assurances. This can result in unexpected inconsistencies in product performance.
Quality and Batch Consistency
Consistency in production is another key factor separating GMP from non-GMP supplements. GMP regulations require manufacturers to maintain detailed records for each batch, covering ingredient amounts, equipment settings, process steps, and quality checks. These records, combined with strict controls over factors like mixing times, temperature, humidity, and capsule weight, ensure that every batch meets the same high standards. By following precise procedures instead of relying on operator memory, GMP-certified manufacturers consistently deliver supplements that meet potency, dissolution, and performance expectations.
In contrast, non-GMP products often lack such structured procedures, leading to variability in quality. For example, a non-GMP herbal supplement might contain significantly less of the active ingredient than stated on the label, whereas GMP-certified products typically maintain much tighter potency ranges.
Transparency and Trust
Transparency is another hallmark of GMP-certified supplements. GMP facilities are required to keep detailed records of ingredient suppliers, batch numbers, test results, equipment cleaning, and product distribution. This level of documentation allows for quick traceability and targeted recalls if safety issues arise. In contrast, non-GMP manufacturers often have incomplete or disorganized records, making it harder to identify and address problems like contamination or mislabeling.
GMP-certified brands often share information about their facilities and display third-party certifications, which builds trust with consumers. Non-GMP brands, however, typically provide minimal insight into their manufacturing processes, leaving customers to rely on marketing claims alone. Independent audits further set GMP-compliant supplements apart by confirming that manufacturers meet recognized quality standards. Products from audited facilities may carry third-party seals, signaling rigorous oversight. Regulatory data has shown that supplement recalls and warning letters are frequently linked to lapses in GMP practices, highlighting the importance of strict manufacturing protocols.
Tools like SlipsHQ are helping to enhance transparency for modern consumers. By scanning a barcode, U.S. shoppers can access information on manufacturing standards, testing practices, contamination risks, and labeling accuracy, all summarized in a science-based trust score. These tools can flag potential issues, such as vague manufacturer details, inconsistent labels, unusually low prices, or a history of recalls - signs of weak GMP controls. On the flip side, clear disclosures about the manufacturing facility, compliance with 21 CFR Part 111, and third-party quality seals are strong indicators of GMP adherence. Together, these transparency measures empower consumers to make informed choices, whether they're shopping in-store or using digital tools like SlipsHQ.
Trust Scores and Consumer Tools for Evaluating Supplements
Navigating the world of supplements can feel overwhelming. With so many options and varying claims, how do you ensure you're making a safe and informed choice? Trust scores and consumer tools simplify this process by turning complex data into straightforward, actionable insights. Let’s break it down.
What Are Trust Scores?
Trust scores are a single, easy-to-understand metric that evaluates a supplement's overall reliability. They take into account key factors like ingredient purity, risk of contaminants, accuracy of labeling, and manufacturing standards. Instead of digging through certifications or ingredient lists yourself, these scores provide a quick way to assess a product’s safety and quality.
Here’s how they work: trust scores combine critical data points such as:
- Ingredient purity: Does the product actually contain what the label claims?
- Contaminant risk: Are there harmful substances present?
- Label accuracy: Is the information provided truthful and complete?
- Manufacturing standards: Does the product meet Good Manufacturing Practices (GMP) and other industry benchmarks?
Some systems even integrate independent testing results or expert evaluations, flagging issues that GMP compliance alone might miss - like hidden allergens or misleading marketing claims.
The National Institutes of Health (NIH) Office of Dietary Supplements highlights that independent organizations test supplements and award seals to those that meet manufacturing and safety standards, such as being free from harmful contaminants. Trust scores take this a step further, combining these certifications with other transparency measures to give you a fuller picture of what you’re buying.
In the U.S., trust scores are designed to align with FDA and NSF standards, use familiar measurement units, and meet local regulations. A reliable trust score system considers compliance with the FDA’s cGMP rule (21 CFR Part 111), third-party certifications, and any FDA warnings or recalls.
Using SlipsHQ to Verify Supplement Safety
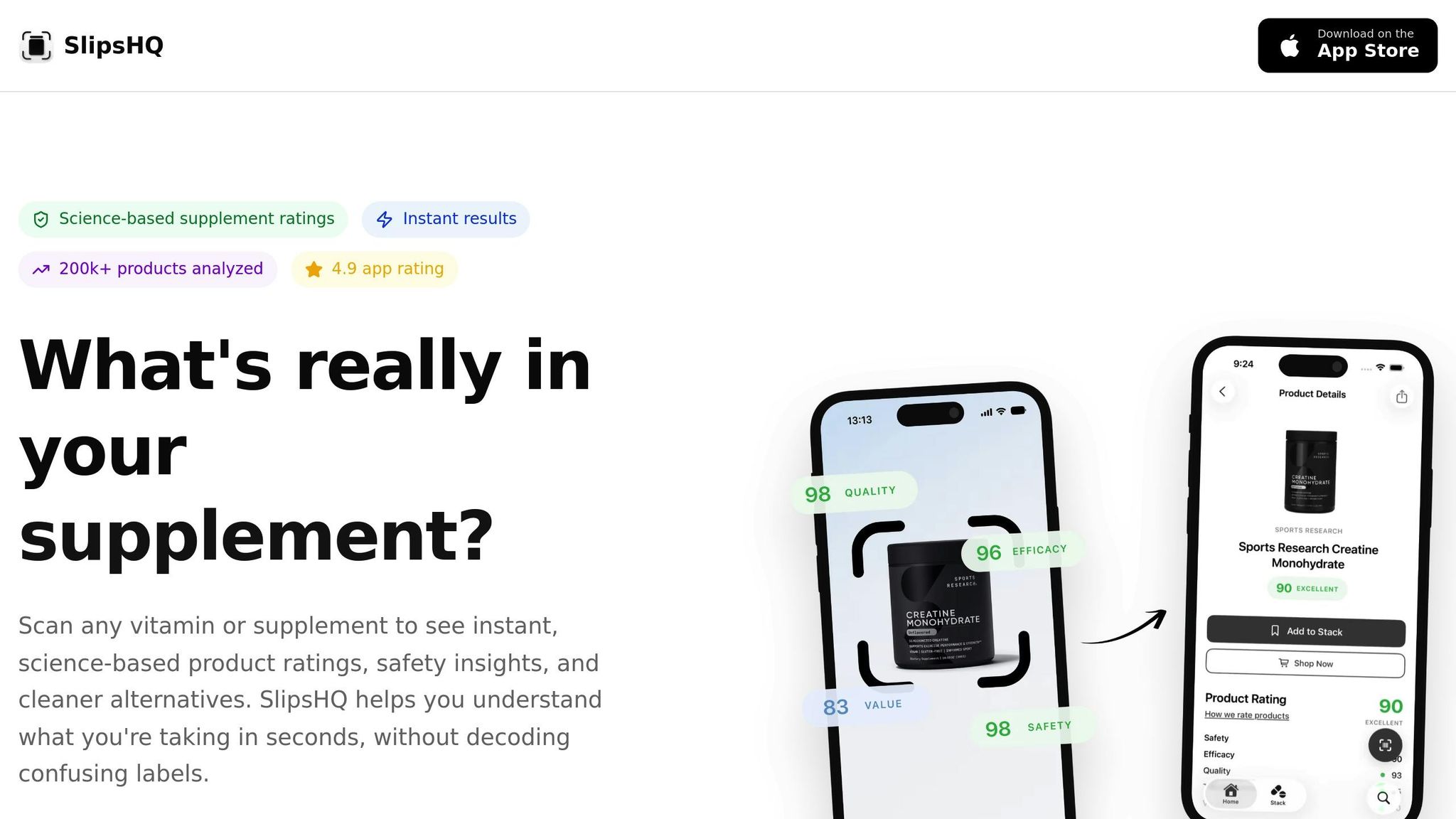
Taking trust scores to the next level, digital tools like SlipsHQ make it even easier to evaluate supplements. SlipsHQ allows U.S. consumers to scan a supplement’s barcode and instantly receive a science-backed trust score on a 0–100 scale. This score is based on a detailed 35-point evaluation system that examines five key pillars:
- Safety
- Efficacy
- Quality
- Transparency
- Value
Each pillar contributes equally to the overall rating, ensuring a balanced assessment.
With a database of over 200,000 supplements from top brands and categories, SlipsHQ provides in-depth ingredient breakdowns, highlights scientific evidence, and flags high-risk components. It’s a powerful tool for anyone looking to make informed decisions.
What makes SlipsHQ stand out is its commitment to independence. The platform doesn’t sell supplements or accept paid placements. Instead, its ratings are based purely on data, certifications, testing practices, and ingredient standards. Additionally, the app lets users compare prices and quality across U.S. retailers, helping you find the best deal without sacrificing safety.
With a 4.9-star app rating and thousands of users across the U.S. and Canada, SlipsHQ has become a go-to resource for health-conscious shoppers seeking clarity and confidence in their supplement choices. The app even offers a 3-day free trial for those who want to test its features.
How to Choose Safe and Effective Supplements
When it comes to supplements, making informed choices is crucial - not just for your wallet but for your health too. By knowing what to look for, you can avoid ineffective products or, worse, those that might pose risks.
Look for GMP-Certified Products
One of the easiest ways to ensure quality is to check for GMP (Good Manufacturing Practices) certification on the label. Certifications from organizations like NSF International or USP (United States Pharmacopeia) indicate that the product has undergone strict inspections and follows standardized production processes. This adds an extra layer of confidence in the product’s safety and consistency.
GMP certification guarantees that each batch of supplements contains the same ingredients in the same amounts, which is especially important for long-term users or those managing specific health concerns. However, while GMP certification ensures manufacturing quality, it doesn’t address whether the ingredients are effective, the dosages are appropriate, or if the claims made by the product are backed by solid research. That’s where additional tools come into play.
Use Tools Like SlipsHQ to Evaluate Ingredients
To go beyond manufacturing standards, consider using supplement evaluation tools like SlipsHQ. These tools analyze the ingredients in a product and provide detailed insights into their safety and effectiveness.
For example, with SlipsHQ, you can scan a supplement's barcode to get a full breakdown of its ingredients. The app explains the purpose of each component, whether it’s supported by scientific evidence, and highlights any potential issues, such as allergens or interactions with medications. This can be especially useful because even GMP-certified products might include ingredients that aren’t suitable for everyone.
SlipsHQ also flags high-risk ingredients and excessive stimulants that may not be immediately obvious from the label. Take a pre-workout supplement as an example - it might be GMP-certified but still contain multiple sources of caffeine that, when combined, exceed safe daily limits. With SlipsHQ, you can catch these details before making a purchase.
What sets SlipsHQ apart is its independence. The platform emphasizes its unbiased approach:
"SlipsHQ does not sell supplements, accept paid placements, or boost scores for brands. Our ratings are based on observable data, certifications, testing practices, and ingredient standards - not sponsorships."
With over 200,000 supplements analyzed and a stellar 4.9 app rating, SlipsHQ has become a trusted resource for U.S. consumers navigating the often confusing supplement market. It’s a powerful tool for ensuring that the products you choose align with your health goals and personal needs.
Conclusion
Opting for GMP-certified supplements is a smart way to prioritize your health and ensure you're getting the value you pay for. These products are made under strict, enforceable standards that guarantee accurate labeling, consistent potency, and minimal contamination risks. On the other hand, non-GMP supplements lack these safeguards, leaving you vulnerable to issues like mislabeling, contamination, and inconsistent quality from one batch to another.
A 2011 FDA report revealed that 73% of inspected supplement manufacturers violated at least one GMP regulation, underscoring the prevalence of quality issues in the industry. By choosing GMP-certified options, you're selecting supplements that undergo thorough testing for identity, purity, strength, and composition at multiple stages of production. This reduces the chances of mislabeled products and costly recalls.
However, GMP certification isn't a one-size-fits-all solution. Even GMP-compliant products might contain ingredients that aren't suitable for you, incorrect dosages, or formulations that could interact with your medications. This is where trust scores come into play. These scores assess supplements based on factors like ingredient transparency, safety warnings, manufacturing quality, and certifications, offering a more complete picture for consumers.
For those navigating the overwhelming array of supplement options in the U.S., tools like SlipsHQ make the decision-making process easier. With a simple barcode scan, you can access vital safety and quality information. SlipsHQ's 35-point evaluation system helps identify top-quality supplements quickly. With over 200,000 supplements analyzed and a stellar 4.9 app rating, it has become a reliable resource for making informed choices in a crowded market.
FAQs
What are the health risks of using non-GMP supplements, and why does GMP certification matter?
Non-GMP (Good Manufacturing Practices) supplements come with certain risks tied to the way they’re made. Without GMP certification, there’s no assurance that the product has been produced under strict guidelines. This opens the door to problems like contamination, incorrect ingredient labeling, or inconsistent dosages - all of which can impact your health.
On the flip side, GMP-certified supplements are manufactured in facilities that adhere to strict safety and quality standards. This certification ensures the product is free from harmful substances, accurately labeled, and consistent in quality. Opting for GMP-certified supplements not only safeguards your health but also gives you confidence in the products you’re using.
What makes GMP-certified supplements safer and higher in quality compared to non-GMP products?
GMP, or Good Manufacturing Practices, certification guarantees that dietary supplements are made following strict quality and safety standards. This certification oversees every step of the process - from sourcing ingredients to the final stages of production - ensuring products are free from harmful contaminants and accurately labeled.
In contrast, supplements without GMP certification don't adhere to these rigorous controls. This lack of oversight can lead to inconsistent quality, mislabeled ingredients, or even the presence of unsafe contaminants. Opting for GMP-certified supplements provides greater peace of mind about their safety and reliability, empowering you to make better choices for your health.
How can SlipsHQ help me find GMP-certified supplements and ensure they’re safe and effective?
SlipsHQ simplifies the process of identifying GMP-certified supplements while offering a clear picture of their safety and quality. By combining barcode scanning with a science-based evaluation system, the app delivers straightforward trust scores and detailed safety insights for each product.
With this tool, you can easily assess ingredient purity, uncover safety warnings, and compare products from different retailers. It’s all about empowering you to make smarter, more confident decisions when it comes to your supplements.
