How Marketing Misleads Supplement Buyers

Marketing in the supplement industry often uses misleading tactics, leaving consumers at risk of wasting money or endangering their health. With no FDA pre-market approval, companies can make exaggerated claims, hide ingredient details, and use deceptive visuals or endorsements to sell products. This lack of oversight creates confusion and leads to unsafe purchases, financial losses, and distrust in the industry.
Key Takeaways:
- Supplements don’t require FDA approval before hitting the market.
- Misleading claims like "miracle cure" or "doctor recommended" often lack strong evidence.
- Labels may hide ingredient amounts or include undisclosed substances, posing health risks.
- Celebrity endorsements and cherry-picked testimonials can falsely boost credibility.
- Tools like SlipsHQ can help verify safety, efficacy, and value before buying.
Stay informed by questioning bold claims, checking for third-party certifications, and using technology to make safer choices.
The Meaningless Claims on Supplement Labels
Marketing Tactics That Mislead Supplement Buyers
Supplement companies often stick to the bare minimum legal requirements while using strategies that can mislead buyers. Understanding these tactics is crucial before making a purchase. Here’s a closer look at how claims, images, labels, and endorsements are often manipulated.
False or Exaggerated Health Claims
One of the most common ways supplement companies mislead consumers is by using scientific-sounding language that lacks solid evidence. Terms like "clinically proven" or "doctor recommended" are thrown around to create a sense of trust, even when the supporting evidence is weak or based on limited studies - sometimes conducted in-house.
Another tactic involves using over-the-top language, such as calling a product a "miracle cure" or describing it as "revolutionary" or an "ancient secret." These words are designed to make the product seem extraordinary. While the FDA prohibits claims that supplements can diagnose, treat, cure, or prevent diseases, companies often use vague structure-function claims like "supports joint health" or "promotes mobility." These phrases can imply more than they actually prove, leaving consumers with inflated expectations.
But misleading claims are only part of the picture. Visuals and language also play a big role in shaping perceptions.
Misleading Language and Images
Visual marketing is a powerful tool, and companies know how to use it to their advantage. For example, those dramatic before-and-after photos you see in ads? They might be digitally altered or influenced by factors like diet or exercise, not just the supplement itself. Descriptive phrases like "prescription strength", "pharmaceutical grade", or "clinical formula" are often used to give the illusion of higher quality or stricter regulation, even when that’s not the case.
Testimonials and reviews are another area where consumers need to tread carefully. While these can offer insight into others' experiences, companies often cherry-pick the most glowing reviews, leaving out less favorable feedback. Some marketing campaigns also play on emotional triggers, addressing concerns like aging or immune health to push buyers toward impulsive decisions, often without fully evaluating the evidence.
And it’s not just the marketing - labels themselves can be misleading or incomplete.
Incorrect Labels and Hidden Ingredients
When it comes to labels, inaccuracies can erode trust. Ingredients listed on the bottle might not match what’s actually inside, whether due to mistakes or deliberate misrepresentation. Proprietary blends are especially tricky, as they hide the exact amounts of individual ingredients, making it hard to assess proper dosages.
Even more concerning is the risk of undisclosed pharmaceutical ingredients, particularly in supplements marketed for weight loss, sexual enhancement, or bodybuilding. These hidden substances can lead to serious health risks, including dangerous interactions with medications or unexpected side effects. While seals like "third-party tested" are meant to reassure consumers, they don’t always guarantee thorough testing. In some cases, they only verify limited aspects, like purity, without addressing potency or ingredient accuracy.
Celebrity and Influencer Endorsements
Endorsements are another way supplement companies sway buyers, often masking the lack of solid evidence behind their claims. Social media has amplified the use of celebrity and influencer endorsements, which can create a false sense of authenticity. Many of these endorsements are part of paid partnerships, and the endorsers may have little to no personal experience with the product. While the Federal Trade Commission requires these relationships to be disclosed, the details aren’t always made obvious.
Even endorsements from healthcare professionals or so-called "experts" can be misleading. Just because a professional backs a product doesn’t mean it’s supported by strong scientific research. Consumers need to critically evaluate all endorsements and look for verifiable evidence to determine whether a supplement is worth their trust.
How Misleading Marketing Affects Consumers
Misleading marketing doesn’t just confuse consumers - it can have real-world consequences that affect health, finances, and trust. Let’s break down the ways these deceptive practices impact people.
Health Risks from Unsafe Supplements
When marketing hides dangerous ingredients or exaggerates safety claims, it puts consumers’ health on the line.
Some supplements have been found to contain undeclared ingredients like banned drugs, heavy metals, or allergens. For instance, weight loss supplements have sometimes included sibutramine, a drug banned by the FDA for its link to serious cardiovascular issues. Similarly, certain sexual enhancement supplements have been found to contain sildenafil or tadalafil - ingredients that can dangerously lower blood pressure, especially when combined with other medications.
Heavy metals like lead, mercury, and arsenic have also been detected in some supplements. These substances can build up in the body over time, leading to neurological or kidney damage.
Another concern is the lack of transparency in proprietary blends, which can result in unsafe consumption of certain nutrients. For example, taking too much vitamin A or iron can lead to severe health problems. And for individuals with food sensitivities, undisclosed allergens in supplements can pose significant risks.
Money Wasted on Ineffective Products
Misleading marketing doesn’t just threaten health - it also hits consumers where it hurts: their wallets.
Many people spend a portion of their income on supplements, expecting results that never materialize. When these products fail to deliver, the financial strain can add up, especially for families already managing tight budgets. Auto-renewal subscriptions and repeat purchases of ineffective products only make matters worse, creating a cycle of wasted money.
To make things tougher, most insurance plans don’t cover supplement costs, leaving consumers to bear the full financial burden.
Loss of Trust in the Supplement Industry
Beyond health and financial concerns, deceptive marketing damages the credibility of the entire supplement industry.
When consumers repeatedly encounter misleading claims, it becomes harder to trust not only the products but also the regulatory bodies meant to oversee them. This distrust makes it challenging for consumers to identify safe, reliable supplements and for ethical companies to stand out in a crowded, often dubious market.
Ultimately, these deceptive practices don’t just harm individuals - they undermine confidence in an industry that many rely on for health and wellness.
How to Spot and Avoid Deceptive Marketing
Navigating the world of supplements can feel like walking through a minefield of exaggerated claims and flashy advertisements. Misleading marketing not only risks your health but can also drain your wallet. Here's how to identify and steer clear of these traps.
Warning Signs in Ads and Product Labels
Certain marketing tactics should immediately raise your suspicion. For example, if a supplement claims to "cure", "treat", or "prevent" diseases, or uses words like "miracle", "instant", or "guaranteed", proceed with caution. Those dramatic before-and-after photos? They’re often manipulated and don’t represent typical results. And celebrity endorsements? They rarely align with scientific evidence.
To ensure you're buying a credible product, look for certifications like NSF, USP, or ConsumerLab on the packaging. These certifications indicate that the product has undergone independent testing for purity and potency. If no such certifications are present, the product may not have been verified.
Another red flag? Proprietary blends that don’t list specific ingredient amounts. Without this information, you can’t know if the doses are effective or if the ingredients might cause unwanted interactions. These labels leave you guessing, which is never ideal when it comes to your health.
Understanding how the FDA regulates supplements can also shed light on what to watch out for.
What FDA Regulations Do and Don't Cover
Unlike prescription drugs, supplements are categorized as food, which means they don’t require pre-market safety or efficacy verification. Here’s what the FDA does - and doesn’t - oversee:
- What the FDA does regulate: The agency can act against supplements that contain harmful ingredients or make illegal health claims. They also enforce basic labeling standards and inspect manufacturing facilities. However, these actions typically occur after an issue has been reported, not before the product hits shelves.
- What the FDA doesn’t cover: The FDA doesn’t test supplements for safety or effectiveness before they’re sold. Nor does it verify that the ingredients listed on the label match what’s inside the product.
Supplement companies also exploit a gray area in marketing. For instance, they can legally say a product "supports immune function" (a structure/function claim) but can’t claim it "prevents colds" (a health claim) without FDA approval. This loophole allows for marketing that, while legal, can still be misleading.
Given these gaps in oversight, relying solely on FDA regulations isn’t enough. That’s where technology can step in to help you make safer, more informed choices.
Using Tools Like SlipsHQ for Better Decisions
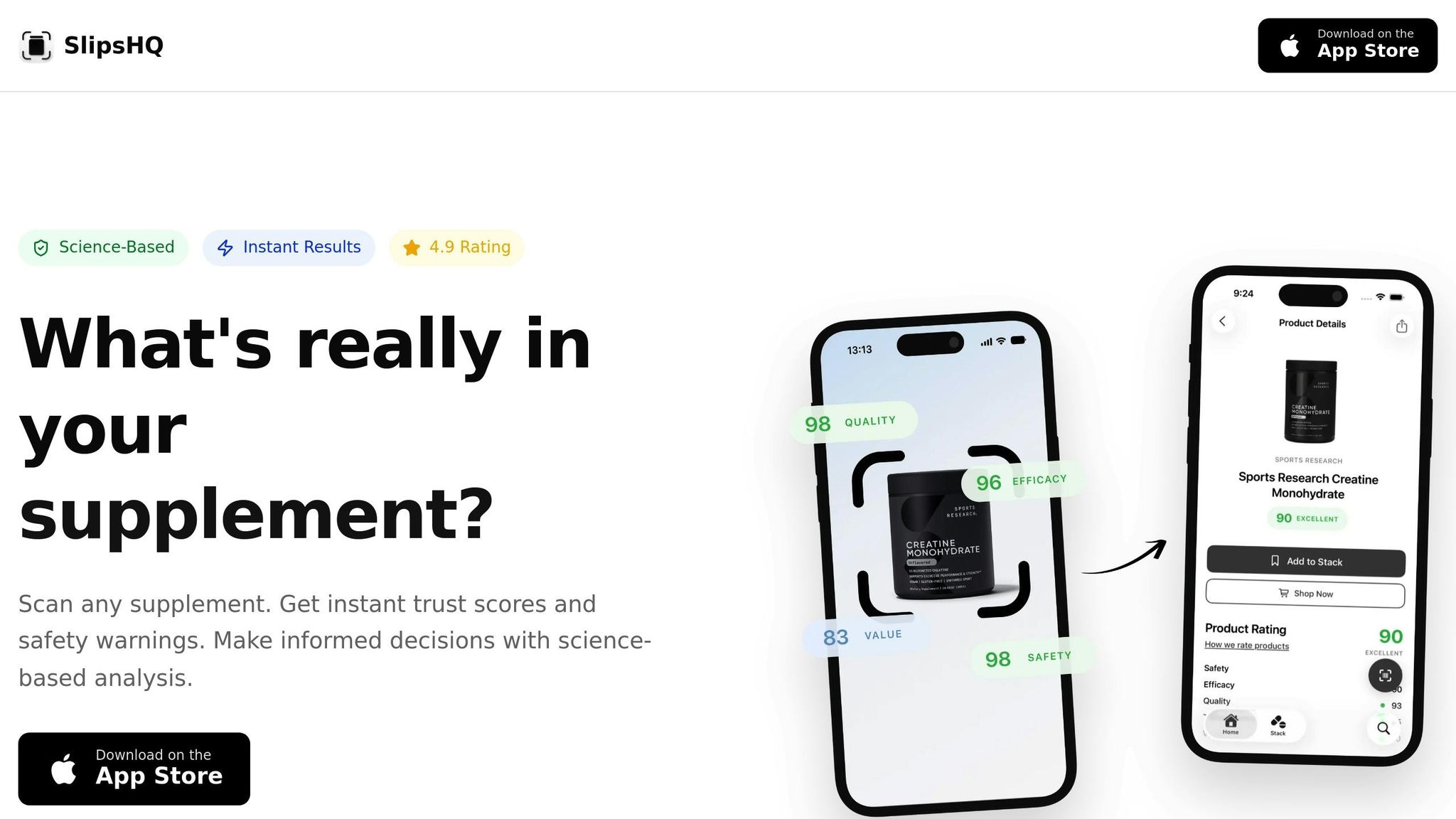
Technology can cut through the noise of marketing claims and hidden ingredients. A great example? SlipsHQ, a mobile app designed to bring clarity to supplement shopping.
With SlipsHQ, you can scan a product’s barcode and instantly access science-backed information. The app assigns trust scores from 0 to 100, evaluating factors like safety, efficacy, quality, transparency, and value. This scoring system simplifies the decision-making process, helping you quickly determine if a supplement is worth your money. Plus, with a database of over 200,000 supplements, it covers most products you’ll encounter.
The app also includes built-in safety warnings, which flag potential drug interactions - especially useful if you’re on prescription medications. It highlights ingredients you might want to avoid based on your health conditions or personal preferences.
Another handy feature? Price comparisons. The app shows you where to find the same product for less, helping you save money over time. And if you’re unsure about combining supplements, SlipsHQ can recommend personalized stacks tailored to your health goals and current routine.
The free version offers basic features like scanning and trust scores, while premium options provide deeper ingredient research and personalized recommendations. Using it is simple: download the app, scan the barcode while shopping, and review the trust score and safety details before making a purchase. It turns what’s often a confusing process into a straightforward, informed decision.
Efforts to Improve Supplement Industry Transparency
The supplement industry has long been criticized for misleading marketing practices, but efforts are underway to bring about change. From regulatory actions to calls for updated legislation, these steps aim to protect consumers and shed light on an often opaque market.
FDA and FTC Enforcement Actions
Federal agencies like the FDA and FTC have ramped up their efforts to hold companies accountable for false claims and unsafe products. The FDA now issues warning letters and recalls for supplements containing undisclosed or illegal ingredients. Similarly, the FTC has increased its focus on deceptive advertising, targeting companies that make unverified promises about weight loss, anti-aging benefits, or disease cures.
However, both agencies face challenges. Limited resources and a reactive approach mean that harmful products often reach consumers before action is taken. Despite these hurdles, these enforcement measures lay the groundwork for broader reforms aimed at improving oversight.
Industry Self-Regulation Efforts
Proposed Policy Changes
Building on enforcement actions, experts are pushing for significant regulatory updates to address gaps in oversight. One major focus is revisiting the Dietary Supplement Health and Education Act of 1994 (DSHEA), which many argue is outdated for today’s market.
One proposed change is mandatory product registration, similar to the system used for prescription drugs. This would allow regulators to track supplements more effectively and respond quickly to safety concerns.
"More stringent FDA oversight, increased enforcement powers, and mandatory registration of supplement products is likely needed to enable proper monitoring of this massive, yet under-regulated industry. Supplement legislation has not been updated since 1994 and is failing to protect consumers from modern-day risks."
- The Ryan Law Group
Other suggestions include requiring pre-approval for advertising to ensure claims are substantiated before they reach the public. Experts also advocate for clear regulations around novel ingredients like CBD, which currently exist in a legal gray area. Additionally, increasing the FDA’s funding and resources could improve its ability to oversee the industry, while stricter penalties for non-compliance would deter bad actors.
These proposed reforms face pushback from industry groups concerned about higher costs and restricted market access. However, growing consumer awareness and high-profile safety issues are driving momentum for change. The challenge lies in finding the right balance between protecting consumers and maintaining room for innovation in the market.
Conclusion: Taking Charge as a Supplement Consumer
With the risks and misleading claims outlined earlier, staying informed is your strongest defense. Be skeptical of any supplement that promises instant miracles or claims to cure illnesses outright.
Thanks to technology, you can fact-check these claims in real time. Tools like SlipsHQ allow you to scan products and access science-backed trust scores instantly. Think of it as turning your smartphone into a personal safety net, helping you steer clear of risky combinations and build a supplement routine that works for you.
While regulations continue to evolve, your best bet is to rely on verified data for every purchase. Each time you buy a supplement, you're casting a vote for companies that value transparency over flashy marketing.
Your health deserves the time and effort it takes to research thoroughly. After all, understanding what you’re putting into your body isn’t just smart - it’s essential for your safety.
FAQs
How can I tell if a supplement's health claims are backed by real science?
To assess whether a supplement's health claims hold up to scrutiny, start by looking for evidence from peer-reviewed research or clinical studies. Claims like "clinically proven" can sound convincing, but without clear references to specific studies, they lack real substance.
It's also important to evaluate the manufacturer's transparency. Reputable companies typically share detailed information about their research, including study results and methodologies. If such details are hard to find, it might be a warning sign.
Tools like SlipsHQ can make this process easier by offering science-backed trust scores and safety insights, helping you make smarter, more informed decisions.
What are the warning signs of misleading or incomplete information on supplement labels?
When you're reading supplement labels, keep an eye out for claims that seem too good to be true - like promises of instant results or miracle cures. Remember, supplements aren't legally permitted to claim they can treat or cure diseases, so any product making such statements should raise a red flag.
Be wary of labels that lack transparency about ingredients or manufacturing processes. Watch out for exaggerated claims or terms like "proprietary blend" that don't specify the amounts of key ingredients. A trustworthy label will clearly list ingredient quantities. Steer clear of products that rely on vague or unproven assertions.
Why aren’t supplements reviewed by the FDA before being sold, and how does this affect their safety and effectiveness?
Dietary supplements, unlike prescription medications, don’t go through FDA review or approval before being sold. This means manufacturers can market these products without first proving they’re safe, effective, or accurately labeled.
Because of this, consumers might come across supplements with misleading claims, ingredients that don’t work, or even substances that could be harmful. To make smart choices, it’s crucial to do thorough research and use trusted, science-backed resources for guidance.
