What Is Stability Testing in Supplements?

Stability testing ensures supplements stay effective, safe, and true to their label claims until their expiration date. It evaluates how ingredients hold up under storage conditions like temperature, humidity, and light. By testing products over time, manufacturers determine accurate expiration dates and identify potential issues like nutrient degradation or unsafe changes. This process uses two main methods:
- Accelerated Testing: Simulates aging quickly by exposing supplements to high temperature and humidity.
- Real-Time Testing: Monitors products under normal conditions over their full shelf life.
Both methods help manufacturers improve formulations, packaging, and storage guidelines, ensuring supplements meet quality standards. For consumers, clear expiration dates, storage instructions, and precise ingredient levels on labels indicate proper testing. Tools like SlipsHQ can also provide quick insights into supplement quality and safety.
Methods of Stability Testing
Supplement manufacturers use two key approaches to assess how their products hold up over time. Together, these methods provide a complete understanding of a supplement's stability.
Accelerated Stability Testing
Accelerated stability testing speeds up the aging process by exposing supplements to higher-than-normal temperature and humidity levels. The product, in its commercial packaging, is placed in an environmental chamber where both temperature and humidity are tightly controlled. These conditions trigger chemical reactions at a faster rate.
In this setup, one month in the chamber can mimic six months of natural aging. This compressed timeline allows manufacturers to quickly gather data on how their supplements might perform over time without waiting years for results. It’s a great way to spot potential stability issues early, enabling adjustments to formulation or packaging before the product hits the market. During testing, samples are analyzed for potency loss and other signs of degradation. However, while this method provides valuable early insights, it’s not a substitute for long-term studies. The extreme conditions in accelerated testing don’t always replicate how supplements degrade in everyday storage.
Real-Time Stability Testing
Real-time stability testing takes a slower, more methodical approach. Here, samples of the packaged supplement are stored in a controlled environment that matches typical storage conditions - think store shelves, warehouses, or even kitchen cabinets. These studies are conducted over the full expected shelf life of the product, which can range from 12 to 60 months, depending on the manufacturer’s claims.
Throughout this period, samples are tested at regular intervals to monitor how well the supplement retains its potency and physical characteristics. This method provides detailed chemical data that confirms ingredient levels over time. Because it reflects natural aging under normal conditions, real-time testing offers the most accurate prediction of a product’s shelf life and storage requirements. The results also form the basis for the expiration dates printed on supplement labels. While real-time studies demand significant time and resources, they are crucial for meeting regulatory standards and ensuring consumer safety.
Comparison of Accelerated and Real-Time Methods
Each method plays a unique role in ensuring supplement quality, but they differ in several important ways:
| Aspect | Accelerated Stability Testing | Real-Time Stability Testing |
|---|---|---|
| Timeframe | Simulates 6 months in a short period | Runs 12 to 60 months |
| Environmental Conditions | High-temperature, high-humidity | Typical ambient storage conditions |
| Purpose | Early insights and quick screening | Definitive shelf-life validation |
| Data Reliability | Preliminary; needs real-time confirmation | Most accurate and regulatory-approved |
| Cost | Lower | Higher |
| Regulatory Use | Supplemental to real-time data | Primary basis for expiration dates |
By combining both methods, manufacturers get the best of both worlds. Accelerated testing identifies potential problems early, allowing for timely adjustments to formulations or packaging. Meanwhile, real-time testing offers the precise data needed to back up expiration dates and comply with regulatory standards. This dual approach ensures that supplements remain safe and effective throughout their intended shelf life while enabling manufacturers to develop products efficiently.
Up next, we’ll explore the key steps and tools involved in the stability testing process.
The Stability Testing Process
Stability testing plays a crucial role in confirming a supplement's shelf life. Through a structured, step-by-step approach, it ensures that products remain safe and effective over time.
Key Steps in Stability Testing
The process starts with selecting representative samples from several batches, all packaged in their commercial containers. These samples are then stored in environmental chambers designed to replicate real-world storage conditions. For accelerated testing, the chambers simulate harsher conditions - typically around 104°F (40°C) with 75% relative humidity - to speed up the aging process. By carefully controlling factors like temperature, humidity, and light exposure, manufacturers can pinpoint how each element impacts product stability.
Testing intervals are established based on the expected shelf life and the type of study being conducted. In accelerated stability testing, samples are analyzed at intervals such as 0, 1, 3, and 6 months to mimic the effects of years of natural aging. Real-time studies, on the other hand, cover the entire shelf life - often ranging from 12 to 60 months, as outlined by regulatory guidelines.
At each interval, manufacturers evaluate chemical potency, physical integrity, microbial safety, and sensory characteristics. This data is then used to create degradation curves, which show how ingredients break down over time. When the potency of an ingredient falls below 90% of its original concentration, manufacturers determine the product's shelf life.
In addition to these tests, manufacturers perform in-use stability studies to assess product quality after the package has been opened. These studies simulate typical household conditions - like exposure to air, moisture, and temperature changes - and provide recommendations such as "use within 6 months of opening" or advice to store the product in a cool, dry place away from sunlight.
Tools and Techniques Used
To evaluate supplement stability, manufacturers rely on a variety of analytical methods. High-performance liquid chromatography (HPLC) and spectrometry are widely used to measure active ingredient levels and identify chemical degradation.
Physical tests assess factors like tablet hardness and capsule integrity, ensuring that the product maintains its intended form over time. Microbiological tests check for bacterial growth or contamination risks, while sensory evaluations focus on changes in appearance, color, odor, and texture.
These techniques provide critical insights into how supplements degrade. They help manufacturers identify ingredients that are prone to breakdown, guiding decisions on formulation adjustments, packaging improvements, and storage recommendations.
How Stability Testing Affects Supplement Quality
Stability testing plays a key role in ensuring the quality, safety, and reliability of supplements. It confirms that the product maintains its stated potency and composition throughout its shelf life, aligning what’s on the label with what’s actually inside the bottle.
Setting Accurate Expiration Dates
The expiration date on a supplement bottle isn’t a random guess - it’s the result of detailed stability testing. This testing simulates how a product ages under typical storage conditions, whether it’s sitting on a store shelf, in a warehouse, or in your kitchen cabinet. For example, if accelerated testing shows a 10% potency loss over a certain period, the expiration date is set to guarantee at least 90% potency until that date. This data-driven approach ensures expiration dates are based on measurable results, not assumptions.
Maintaining Ingredient Potency Over Time
Beyond determining expiration dates, stability testing helps manufacturers ensure that ingredient potency remains intact. Factors like temperature, humidity, light, and oxygen exposure can cause ingredients to degrade at different rates. By analyzing how these elements affect the product, manufacturers can identify vulnerable ingredients and take steps to improve stability. This might involve tweaking ingredient ratios, using more stable ingredient forms, or enhancing packaging to better protect the product.
These efforts ensure that the active ingredients listed on the supplement facts panel remain in the promised amounts until the expiration date. This not only guarantees the product’s effectiveness but also prevents issues like underdosing, which could compromise the supplement’s benefits.
Reducing Product Recall Risks
Stability testing also helps manufacturers avoid costly recalls by identifying potential issues before products hit the market. For instance, if a key ingredient loses 50% of its potency halfway through the shelf life, or if tablets start crumbling or showing signs of microbial growth, a recall might be necessary. By addressing these problems early, manufacturers can fine-tune formulations, choose better packaging, and provide accurate storage recommendations, such as “store in a cool, dry place” or “keep away from direct sunlight.”
This proactive approach ensures a safer, more reliable product and builds consumer trust. When manufacturers openly share their stability testing practices, it reassures customers that the supplements they’re buying have been thoroughly evaluated to deliver the promised benefits throughout their shelf life. This transparency empowers consumers to make more informed decisions when choosing supplements.
Using Stability Testing Knowledge as a Consumer
Understanding stability testing isn’t just for manufacturers - it’s a tool you can use to make smarter decisions when buying supplements. While the technical testing happens behind the scenes, labels can offer valuable clues about whether a product has been properly tested and will live up to its claims. Here’s how to use that information to your advantage.
Reading Quality Indicators on Labels
A clear expiration date is one of the first things to check. It signals that the supplement has undergone proper stability testing. If you see a vague "best by" timeframe or no expiration date at all, consider it a warning sign that the product may not have been adequately tested.
Storage instructions like "store in a cool, dry place" are based on stability testing data. When these details are included, it shows the manufacturer has studied how environmental conditions - like temperature and humidity - affect the product's ingredients.
The supplement facts panel is another key area to inspect. Manufacturers confident in their testing will list precise potency levels for active ingredients, showing that those levels are expected to remain stable until the expiration date. Look for specific measurements like "500 mg Vitamin C" instead of vague claims. Also, check for third-party testing certifications, which further validate the product’s quality.
Batch numbers and manufacturing dates can provide insight into a product’s shelf life. For example, a supplement produced in January 2025 with an expiration date of January 2027 indicates a 24-month stability. While supplements within their expiration window are generally safe, fresher products might retain slightly higher potency.
Be cautious with supplements boasting extra-long expiration dates - like five years or more - unless the label explains why. Most supplements are designed to last between 12 and 60 months. Also, inspect the packaging for any signs of damage, discoloration, or deterioration, as these could mean the product has been exposed to conditions that compromise its stability. Once purchased, store supplements as directed on the label and, if possible, in their original packaging. The packaging is often chosen specifically to protect the product based on stability testing results.
But what if the label doesn’t give you enough confidence? That’s where technology can step in.
How SlipsHQ Helps You Understand Supplement Quality
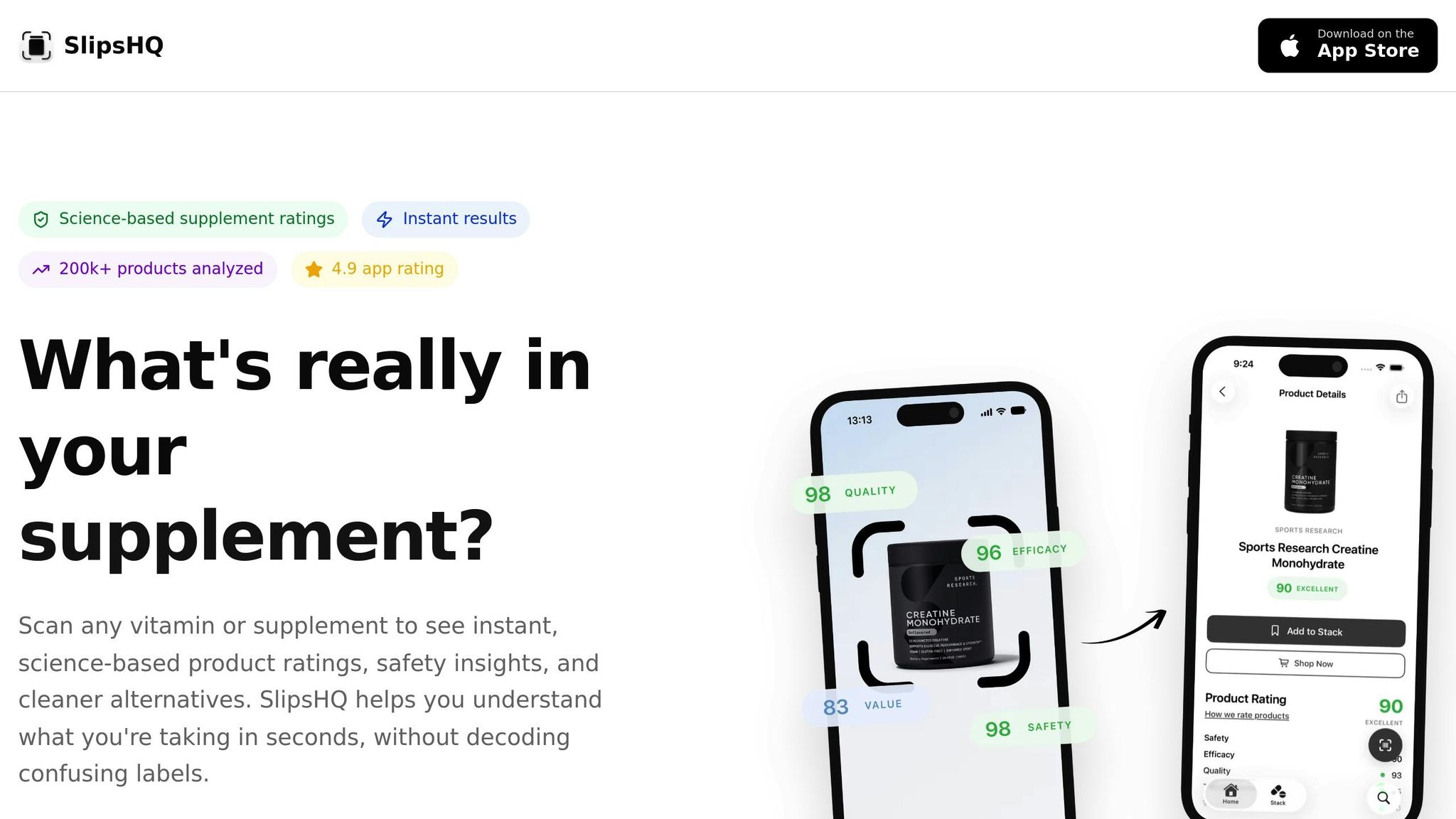
Deciphering supplement labels can feel overwhelming, especially when comparing multiple products. That’s where SlipsHQ, a user-friendly app, comes in. It offers quick, science-backed insights into the quality, safety, and effectiveness of supplements - factors directly linked to stability testing.
With SlipsHQ, you can scan a product’s barcode and instantly receive a detailed rating on a scale from 0 to 100. This rating is based on a 35-point evaluation system covering five essential pillars: Safety, Efficacy, Quality, Transparency, and Value. The Quality pillar, for instance, incorporates testing practices and ingredient standards, reflecting the same principles behind stability testing.
The app also provides an in-depth breakdown of ingredients, explaining their purposes, scientific support, and any potential concerns. You’ll get alerts for safety risks or ingredient interactions, which can arise from unstable formulations or improper storage. With a database of over 200,000 supplements and a stellar 4.9 rating, SlipsHQ delivers independent assessments based solely on objective data.
For those who want to make informed choices without digging into technical details, SlipsHQ simplifies the process. The app offers a 3-day free trial, and afterward, you can subscribe for $4.99 per week or $39.99 per year. By translating stability testing metrics into actionable insights, SlipsHQ helps you identify supplements that maintain their safety and potency throughout their shelf life - exactly what you need to make confident, informed purchases.
Conclusion
Stability testing is the cornerstone of ensuring that supplements live up to their claims - from the day they’re manufactured to their expiration date. Without this critical process, there’s no way to guarantee that a supplement purchased today will maintain its potency and quality over time. It’s through stability testing that manufacturers can confirm a product’s potency, purity, and overall integrity throughout its shelf life.
For manufacturers, this testing isn’t just about meeting quality standards - it’s about prioritizing consumer safety. It helps reduce product recalls, avoid legal complications, and build trust with customers. By identifying which nutrients are prone to degradation and analyzing how factors like packaging, storage, and time impact a product, manufacturers can determine accurate expiration dates and provide practical storage guidelines.
But stability testing doesn’t just benefit manufacturers - it also empowers you, the consumer. Knowing about this process can help you make better choices when buying supplements. Look for clear expiration dates, detailed storage instructions, and precise potency information on labels. If the label feels vague or incomplete, tools like SlipsHQ can simplify the science. This app decodes complex stability data into actionable insights using barcode scanning and offers ratings based on safety, quality, transparency, and value. It’s an easy way to identify products from brands that prioritize rigorous testing, ensuring the supplements you buy remain effective throughout their shelf life.
In short, stability testing transforms supplement shopping into a more informed and reliable experience, making sure that what’s promised on the label is exactly what you get.
FAQs
What is stability testing, and why is it important for supplements?
Stability testing plays a key role in ensuring that a supplement retains its quality, potency, and safety throughout its shelf life. This process examines how ingredients break down over time and assesses the product's ability to handle environmental factors like temperature shifts, humidity, and light exposure.
The goal is to confirm that supplements remain effective and safe for the entire period they're intended to be used. By catching potential problems early, stability testing allows manufacturers to consistently produce reliable, top-tier products. Tools like SlipsHQ also help consumers by offering clear, detailed insights into a supplement's safety and effectiveness, making it easier to choose products with confidence.
How do supplement manufacturers decide expiration dates using stability testing?
Stability testing is a methodical process used by manufacturers to figure out how long a supplement stays safe and effective when exposed to certain conditions. By examining elements like temperature, humidity, and light, they can estimate how the product's ingredients break down over time.
This process ensures that the expiration date on the label accurately represents the supplement's quality and potency until that point. For those who prioritize their health, stability testing is essential in providing confidence in the product’s safety and performance.
How can I tell if a supplement has undergone proper stability testing?
To confirm that a supplement has been properly tested for stability, look for labels that highlight compliance with Good Manufacturing Practices (GMP) or reference specific stability testing protocols. These tests ensure the product retains its effectiveness, safety, and quality over time when stored as recommended.
Pay attention to key details such as expiration dates, storage instructions (like "store in a cool, dry place"), and certifications from trusted third-party organizations. These factors often signal a product that has undergone thorough testing. If you're unsure about a supplement's quality, tools like SlipsHQ can provide science-based insights to help you make an informed decision.
